Today's News: FDA Narrows Covid Vaccine Eligibility to High-Risk Groups
New vaccines for healthy adults and children will now require placebo-controlled clinical trials before approval.
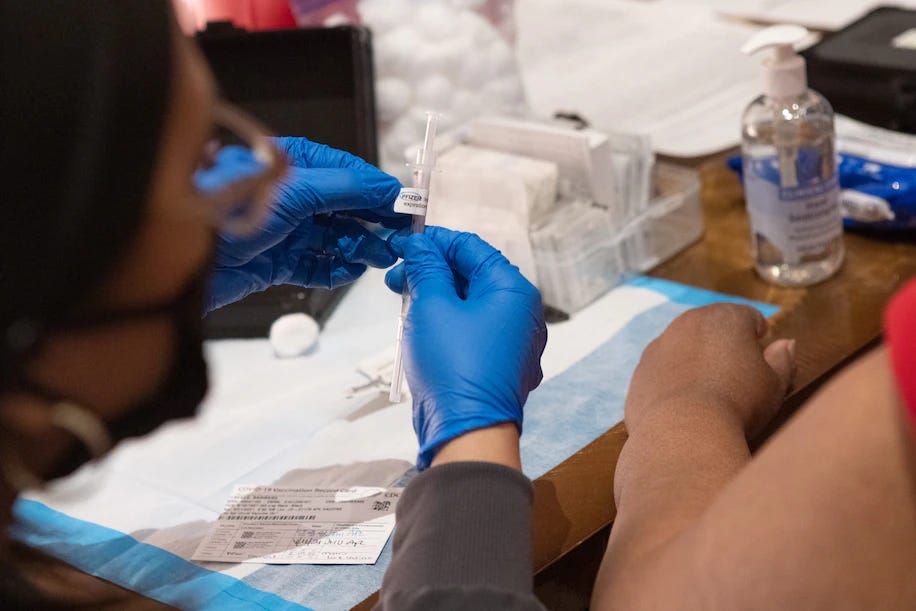
Photo: Allison Shelley/For The Washington Post
Overview
Date: May 20, 2025
Topic: FDA Narrows Covid Vaccine Eligibility to High-Risk Groups
Summary: The U.S. Food and Drug Administration announced a significant policy shift limiting Covid-19 vaccine access to adults 65 and older and individuals of any age with high-risk medical conditions. New vaccines for healthy adults and children will now require placebo-controlled clinical trials before approval. Officials say the aim is to rebuild public trust and align U.S. policy with other countries, but critics warn the change could restrict access, reduce uptake, and politicize vaccine regulation. The revised framework follows the appointment of vaccine skeptics to key roles in the Trump administration and has sparked debate among experts and public health advocates.
Sources
The New York Times: F.D.A. Poised to Restrict Access to Covid Vaccines
CNN: FDA to limit future Covid-19 shots to older people and those at risk of serious infection
NBC News: FDA says Covid vaccines likely not available for healthy kids and adults this fall
The Washington Post: FDA to limit covid shot approval to elderly, those with medical conditions
The Wall Street Journal: Trump Administration Toughens Requirements for Covid Vaccine Approval
Associated Press: New Trump vaccine policy limits access to COVID shots
Key Points
The FDA will restrict Covid-19 vaccine approval this fall to adults 65+, pregnant women, and individuals with qualifying medical conditions such as diabetes, asthma, or obesity.
Approval for healthy individuals under 65 will now require full placebo-controlled trials showing clear clinical benefit.
The shift is presented as a return to a more evidence-driven, risk-based framework, consistent with vaccine policies in Europe and Canada.
Officials claim the policy aims to restore trust in vaccines after public resistance to broad mandates.
CDC estimates suggest up to 200 million Americans may still qualify under the new criteria.
The announcement comes amid increased political influence in health agencies following appointments by the Trump administration.
Unique Highlights
CNN emphasized that the policy shift excludes consideration of long Covid and noted low booster uptake in recent years as a motivation for the change.
NBC News reported that the new trial requirements will delay or prevent a fall rollout for healthy children and adults, citing logistical hurdles for drugmakers.
The New York Times quoted FDA officials calling the move a "reasonable compromise" and detailed criticisms from frontline physicians warning of unnecessary deaths.
The Washington Post revealed internal FDA deliberations and noted that insurers may not cover vaccines for lower-risk individuals unless the CDC makes permissive recommendations.
The Wall Street Journal stressed industry concerns that large new trials could chill investment in Covid vaccine development.
Associated Press included comments from the American Academy of Pediatrics warning of negative impacts on access for children and families.
Contrasting Details
CNN and The Wall Street Journal framed the FDA’s shift as a scientific recalibration, while The New York Times, AP, and NBC News highlighted the political overtones tied to appointments of vaccine skeptics like Robert F. Kennedy Jr.
The Washington Post and NBC News raised concerns about the FDA overriding the CDC’s traditional role in determining vaccine eligibility, whereas The Wall Street Journal presented the FDA’s authority as a return to rigorous standards.
The New York Times and AP noted skepticism around observational data used in prior vaccine efficacy studies, while CNN and The Washington Post cited experts defending the current safety and effectiveness evidence.
NBC News reported that drugmakers may not meet trial timelines before fall, while FDA officials quoted by The Wall Street Journal and CNN suggest the high-risk group vaccines will proceed using existing immunogenicity data.
The Newsie Project uses AI to summarize, compare, and contrast the reporting of the major US and world online news sources.
This is an evolving project. Tools, approaches, and output formats will change over time. The Newsie Project does not attempt to provide a definitive capsule of any news story. While the incidence of errors in these summaries is low, and I attempt to spot-check details, AI tools can hallucinate. Please click through and read the articles for details (some may be paywalled).